4. Clarification on reporting requirements: Guideline for the notification of serious breaches of Regulation (EU) No 536/2014 or the clinical trial protocol
-
Serious breaches which occurred outside the EU/EEA while the application for a clinical trial authorisation (CTA) is submitted but not yet authorised in the EU/EEA territory and the serious breach has an impact on the safety and/or the rights of a trial participant or reliability and robustness of data filed in an application dossier, the sponsor should address the concerns during the evaluation of the CTA. If this is not feasible or not satisfactory, this might lead to the withdrawal of the application via CTIS. If for example the serious breach resulted from flaws in the design of the clinical trial, the CTA may need to be withdrawn
-
Serious breaches of an EU/EEA authorised clinical trial occurring exclusively outside the EU/EEA that are likely to affect the safety and/or the rights of a trial participant or the reliability and robustness of the data generated in a clinical trial already authorised or being conducted in the EU/EEA territory, should be notified to the MSC via the CTIS under the reporting requirement of Article 52.
-
When a sponsor notifies a serious breach, they should also consider if there are any other relevant notifications that need to be undertaken to comply with the Regulation (EU) No 536/2014, for example, requirements under Article 53 for unexpected events, or under Article 54 for urgent safety measures, or substantial modifications following a temporary halt or the decision to early terminate the trial under Article 37 and Article 38, respectively.
All relevant fields in CTIS, as presented in Appendix IIIa of this document must be completed. Unless the information reported in the CTIS fields is deemed exhaustive, the sponsor is encouraged to upload a report (using the “Add document” bottom) which should include all the details needed for AMSs/MSCs to assess the reported serious breach. To this purpose, the Appendix III b lists the topics which are expected to be covered. Sponsors should update the serious breach details in the CTIS fields and/or with a follow-up report if new information becomes available.
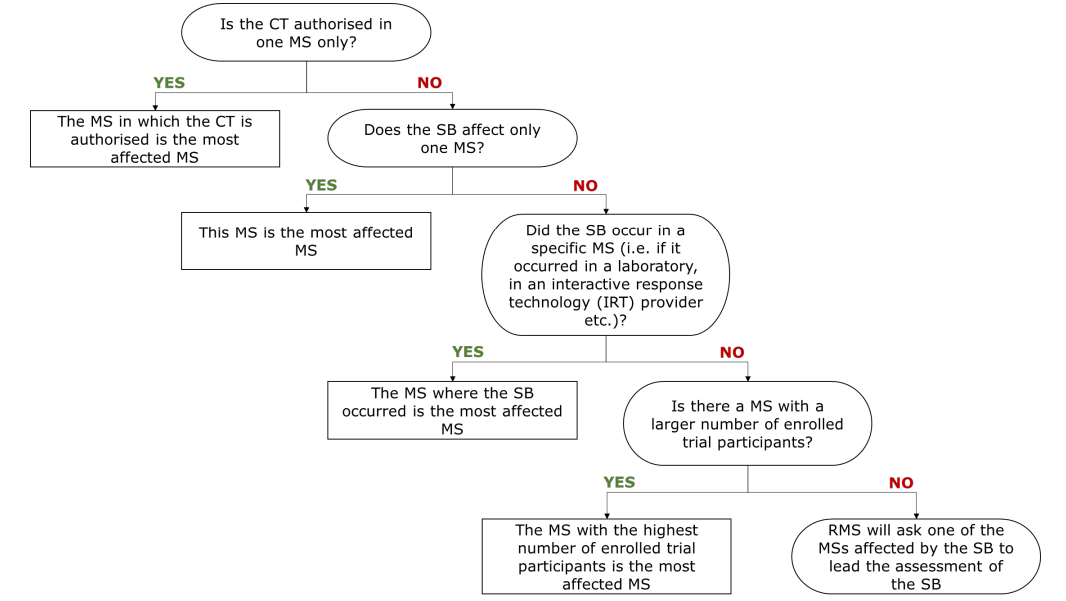
The sponsor should follow the below diagram and indicate which is the Member State most affected by the serious breach reported in the title of the notification and should report more details in Appendix III b.
© European Medicines Agency, 2021
Clinical Research News
Upcoming Clinical Research Training and Conferences
-
on demand
 ACROSS Global Training Academy
Online
ACROSS Global Training Academy
Online -
29 September 2023 – 02 October 2024
 SCDM (Society of Clinical Data Management)
United States
SCDM (Society of Clinical Data Management)
United States -
16 – 18 April 2024
 Informa
United States
Informa
United States -
17 – 19 April 2024
 SCDM (Society of Clinical Data Management)
Poland
SCDM (Society of Clinical Data Management)
Poland -
21 – 23 May 2024
 Informa
Belgium
Informa
Belgium -
03 – 04 June 2024
 The Society for Clinical Research Sites (SCRS)
United States
The Society for Clinical Research Sites (SCRS)
United States -
16 – 17 July 2024
 The Society for Clinical Research Sites (SCRS)
Australia
The Society for Clinical Research Sites (SCRS)
Australia -
27 September – 29 October 2024
 The Society for Clinical Research Sites (SCRS)
United States
The Society for Clinical Research Sites (SCRS)
United States
Upcoming Clinical Trials
-
Universidad de CuencaRecruiting
-
Suleyman Demirel UniversityNot yet recruitingPremenstrual Syndrome | Family Characteristics | Exercises | Stress LevelTurkey
-
Assiut UniversityNot yet recruiting
-
Institut Claudius RegaudLaboratoire IJCLabNot yet recruiting
-
Chugai PharmaceuticalNot yet recruitingAntiphospholipid Syndrome (APS) | Immune Thrombocytopenia (ITP) | Dermatomyositis (DM) | Bullous Pemphigoid (BP) | Behçet's Syndrome (BS) | Immune-mediated Necrotizing Myopathy (IMNM)United States, Japan
-
Universität MünsterNot yet recruiting
-
Mỹ Đức HospitalNot yet recruitingHormone Replacement Therapy | Embryo Transfer | Letrozole | Irregular Menstruation
-
Wageningen UniversityRecruitingBlood Pressure | Cardiometabolic HealthNetherlands
-
Peking UniversityRecruitingConditions or Focus of Study: B7-H3 Positive Relapsed/Advanced Malignant Solid TumorChina
-
Universidade do PortoInstituto Portugues de Oncologia, Francisco Gentil, Porto; Unidade de Saúde... and other collaboratorsNot yet recruitingPapillomavirus Infections | Cervix Cancer | Early Detection of Cancer | Self-Examination
-
Qilu Pharmaceutical Co., Ltd.Not yet recruiting
-
The Second Affiliated Hospital of Chongqing Medical...RecruitingPostoperative Nausea and Vomiting | Stellate Ganglion Block | Thyroid SurgeryChina
