Glossary: Guideline for the notification of serious breaches of Regulation (EU) No 536/2014 or the clinical trial protocol
Clinical Trial: means a clinical study which fulfils any of the following conditions: (a) the assignment of the trial participant to a particular therapeutic strategy is decided in advance and does not fall within normal clinical practice of the Member State concerned; (b) the decision to prescribe the investigational medicinal products is taken together with the decision to include the trial participant in the clinical study; or (c) diagnostic or monitoring procedures in addition to normal clinical practice are applied to the trial participants.
Sponsor: means an individual, company, institution or organisation which takes responsibility for the initiation, for the management and for setting up the financing of the clinical trial. For the purpose of this guideline, the sponsor can officially nominate authorised delegates. This authorised delegates nominated by the sponsor can be, for example, a legal representative or contract research organisation (CRO).
Service provider: means a party involved in the trial, for example, a CRO or other contracted organisation, with clinical trial related responsibilities delegated by the sponsor, other than the “delegated party” and “investigator”, which could observe a breach and is requested to report it to the sponsor/delegated party; it can be, for example, a CRO, a vendor responsible for the interactive response technologies (IRT), the site/sites involved in the manufacturing of IMP.
Delegated party: a type of service provider, is a party delegated by the sponsor, through a written contract, to perform the tasks set out in Article 52 of Regulation (EU) No 536/2014, i.e. to assess incidents which could be suspected serious breaches and/or to report suspected serious breaches to CTIS on behalf of the sponsor.
Delegation of tasks does not remove the ultimate responsibility of the sponsor or investigator for the conduct of the clinical trial in accordance with the applicable legislation. Investigator: means an individual overall responsible for the conduct of a clinical trial at a clinical trial site. If a trial is conducted by a team of individuals at a trial site, the investigator is the responsible leader of the team and may be called the Principal Investigator.
Suspected serious breach: means an incident which at the time of communication from investigators or from service providers to the sponsor has not yet been assessed by the sponsor to be a serious breach.
Serious breach: Any deviation of the approved protocol version or the clinical trial regulation that is likely to affect the safety, rights of trial participants and/or data reliability and robustness to a significant degree in a clinical trial.
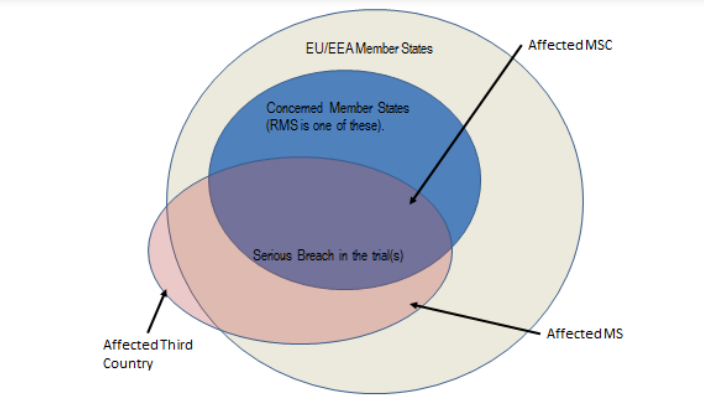
Member State Concerned (MSC): means the Member State where an application for authorisation of a clinical trial or of a substantial modification has been submitted under Chapters II or III of the Regulation (EU) No 536/2014 respectively.
Reporting Member State (RMS): is the Member State Concerned elected in line with requirements of Article 5 of the Regulation (EU) No 536/2014, in the lead for the validation and assessment of part I phases.
© European Medicines Agency, 2021
Clinical Research News
Upcoming Clinical Research Training and Conferences
-
on demand
 ACROSS Global Training Academy
Online
ACROSS Global Training Academy
Online -
29 September 2023 – 02 October 2024
 SCDM (Society of Clinical Data Management)
United States
SCDM (Society of Clinical Data Management)
United States -
21 – 23 May 2024
 Informa
Belgium
Informa
Belgium -
03 – 04 June 2024
 The Society for Clinical Research Sites (SCRS)
United States
The Society for Clinical Research Sites (SCRS)
United States -
16 – 17 July 2024
 The Society for Clinical Research Sites (SCRS)
Australia
The Society for Clinical Research Sites (SCRS)
Australia -
27 September – 29 October 2024
 The Society for Clinical Research Sites (SCRS)
United States
The Society for Clinical Research Sites (SCRS)
United States
Upcoming Clinical Trials
-
National Institute of Medical Sciences and Nutrition...Not yet recruitingRhA - Rheumatoid Arthritis
-
Institute of Liver and Biliary Sciences, IndiaNot yet recruitingAcute-On-Chronic Liver FailureIndia
-
Centre Leon BerardNot yet recruitingGastric Cancer | Oesogastric Junction CancerFrance
-
TC Erciyes UniversityRecruitingEndodontic DiseaseTurkey
-
Casa di Cura Privata 'Malzoni - Villa dei Platani...Recruiting
-
Assembly BiosciencesNot yet recruitingRecurrent Genital Herpes Simplex Type 2New Zealand
-
Cerenovus, Part of DePuy Synthes Products, Inc.Not yet recruitingAcute Ischemic StrokeUnited States
-
Pamukkale UniversityNot yet recruitingUrinary Bladder, OveractiveTurkey
-
China-Japan Friendship HospitalNot yet recruitingObesity | Diabetes | Gallstones | Biliary Stasis
-
AstraZenecaDaiichi SankyoNot yet recruitingMetastatic Breast Cancer | HER2-positive Breast Cancer | Unresectable Breast Cancer | HER2-low Expressing Breast CancerCanada
-
Chinese University of Hong KongRecruiting
-
Central Hospital, Nancy, FranceNot yet recruiting
